Once a practical synthetic route has been demonstrated and the critical process research required for scale-up has been performed, Acceledev can produce RSM intermediates or APIs under cGMP-like(RSM approved) guidelines.
Our extensive manufacturing capabilities feature flexibility, reliability and quality, in support of any phase of our API program.
Below are some of the key aspects of our work:
- Custom manufacturing of unique New Chemical Entities (NCEs) for Phase I, Phase II, and III API programs




- Process optimization and validation under time pressure
- Lead R&D chemists working seamlessly together with production chemists on the same site
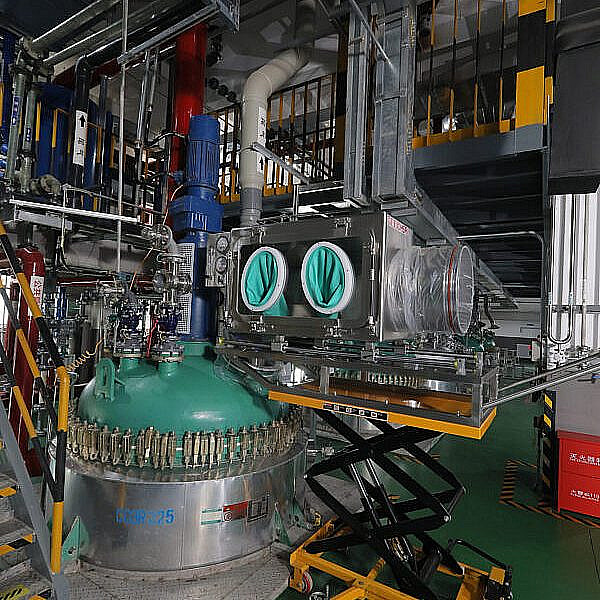
- Multi-purpose 50L to 5000L vessels, with output of kilo to MT batch sizes
- Manufacturing to support commercial launch and post launch supply
- Multi-purpose diverse range of pilot plant and kilo lab facilities; 30L to 300L vessels
- Multiple operation units to undertake a broad range of unique chemistries at all scales
- Fast, efficient implementation of any customer provided technologies.
Reliability
- Professional/experienced production management team, process chemistry team & EHS team working hand-in-hand.
- Professionally designed multi-purpose facilities able to efficiently manage the daily changing project flow.
- Facilities located in stable national and provincial level chemical industrial parks, with complete infrastructure and policy support
- Process safety must be assessed for each scale-up project before moving into the workshop
Quality Assurance
- RSM compliant, ISO9001 certified
- Thoughtfully followed OHSAS18001 and ISO14001 instructions to build EHS management system and SOPs
- Wide range of successful audits from top western pharmaceutical and biotech companies and third-party authorities
